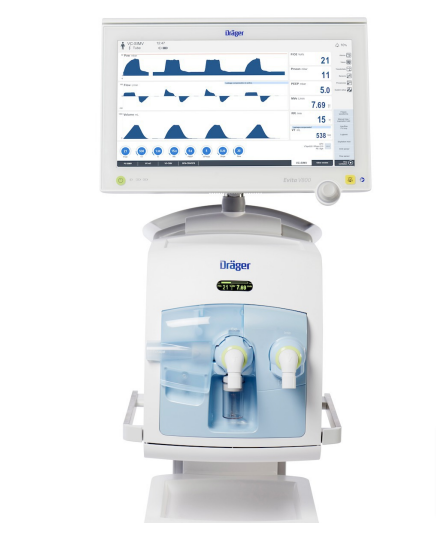
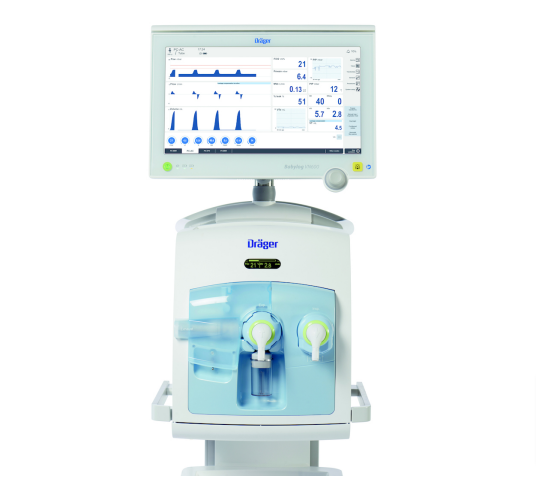
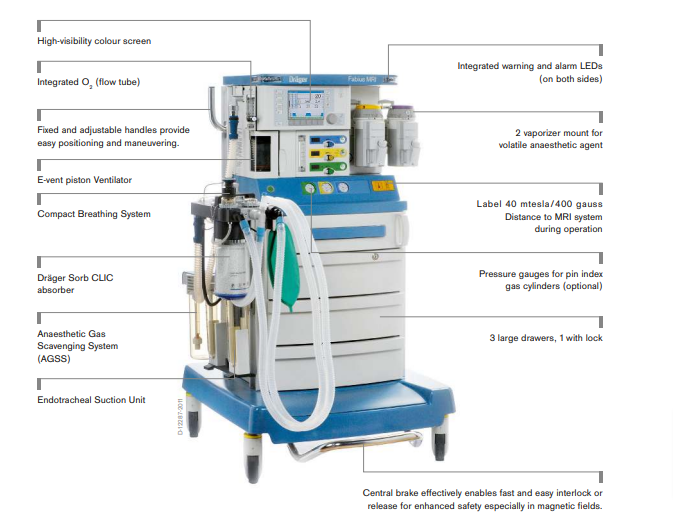
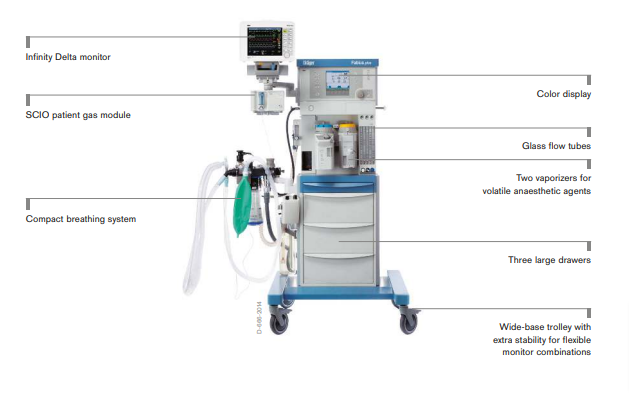
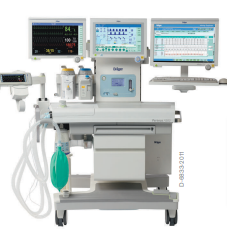
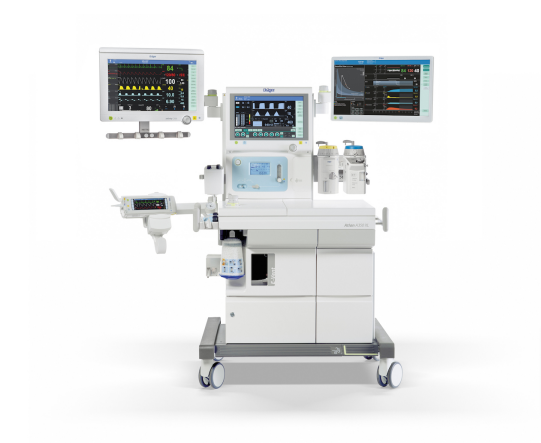
The new platform offers flexibility for most spatial conditions. The high precision piston ventilator supports lung protective ventilation measures and a comprehensive set of parameters assist decision-making support. The Atlan A350/XL can be networked to communicate bi-directionally and
Benefits
Lung Protective Ventilation
The electronically controlled electrically driven piston ventilator technology of Atlan A350/XL anaesthesia machine helps to deploy lung protective ventilation measures that can be beneficial for perioperative lung function and may improve outcomes.
— Synchronised piston movement with the patient expiration flow reduces the expiratory resistance and can reduce work of breathing
— The set PEEP is maintained even in case of small leakage and during spontaneous breathing to reduce risk of atelectasis development
— High trigger sensitivity can detect even weak spontaneous breathing efforts of patients
— Fresh-gas decoupling ensures that changes to the fresh-gas flow have no influence on the applied tidal volume, ventilation pressures, and the accuracy of delivered VT even with very small VTs, e.g. down to 5 ml
— Features and functionalities optimise minimal- and low-flow application, which can contribute to improved humidity of anaesthetic gases, mucociliary clearance, maintenance of body temperature and reduced fluid loss. They include:
— integrated active heating of breathing system to warm breathing gas and to reduce condensation — optimised breathing system architecture to enable fast changes in fresh-gas and agent concentrations — sample-gas recirculation to eliminate gas loss
— Lung recruitment maneuvers option* comprises one-step and multi-step recruitment methods, Insp./Exp. Hold function and reminder function to support recruitment manoeuvre deployment
— AutoFlow option ensures the delivery of the set tidal volume with the lowest required pressure to avoid pressure peaks and unintentional high tidal volumes
— Highly accurate APL valve with a nearly linear increase and decrease in pressure pattern
* this requires software 2.0 or higher
Decision Support
To support you and your staff make informed decisions, our Atlan A350/XL anaesthesia machine can be fitted with multiple options and combinations with other Drager products.
— Advanced Gas Monitoring option*:
— Indicator and trend for efficiency of fresh-gas setting and anaesthetic agent consumption (Econometer and Low Flow Wizard (with no trend)) to support intuitive and convenient application of minimal- and low- flow anaesthesia
— Access to gas and oxygen consumption and anaesthetic agent uptake data to analyse the low- and minimal-flow practices
— MV x COp parameter to monitor the qualitative display of CO9 elimination
— Advanced Ventilation Monitoring option:
— Display of patient lung compliance with trend, P-V and V-Flow loops to assess the ventilation quality and adapt ventilation settings accordingly
-— Compilation of relevant ventilation and haemodynamic patient data in one view display to assess therapeutic effects of lung recruitment manoeuvre** �04 | Drager Atlan® A350/A350 XL Benefits Guidance for optimised and patient oriented anaesthetic agent delivery in combination with Drager’s SmartPilot® View ***
* only with integrated patient-gas measurement module
** only with Drager Infinity? Acute Care System (IACS) patient monitoring
wa software requires Medical Grade PC
Infection Prevention and Control
Breaking the chain of infection and complying with your hospital’s hygiene protocols is critical in today’s clinical environment. For this reason, during the development phase of Atlan anaesthesia machines, we designed them with infection prevention regulations in mind to support hygiene measures in the OR.
Tool-free and quick disassembly of breathing system with few parts to be compliant with infection prevention regulations
Smooth and rounded surfaces ease cleaning/wipe disinfection
Cable ducts and channels reduce number of potential contamination sources
Compatible with original Drager single-use consumables support hygiene standards
Generated message* reminds personnel about the replacement of the RFID technology-based consumables (Infinity ID breathing circuit, Infinity ID WaterLock 2 water trap, Infinity ID flow sensors, Infinity ID CLIC absorber) when their maximum period of use are exceeded
Compliant with ISO 17664
* with option Infinity ID Accessories Support
Workflow Efficiency
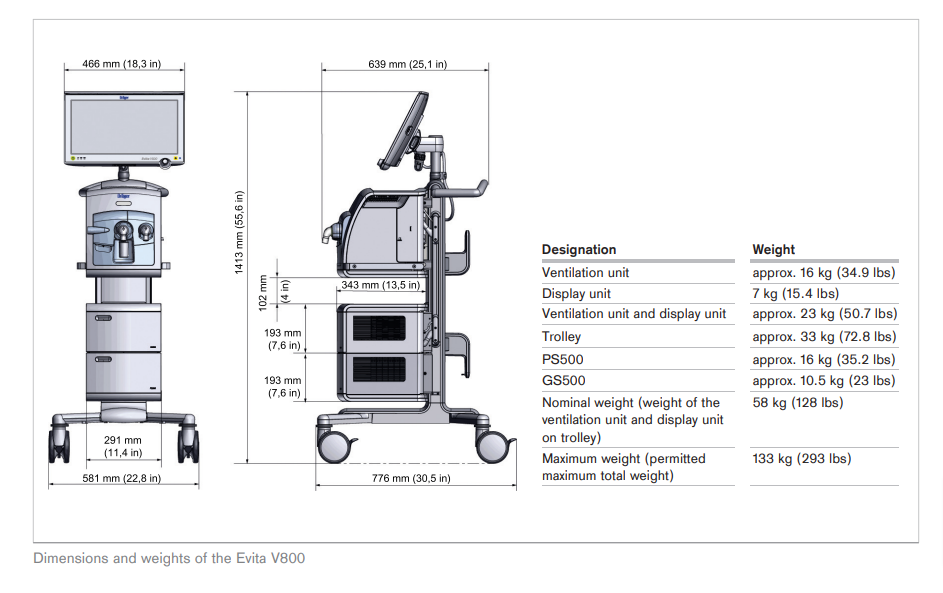
The design architecture of the Atlan A350/XL anaesthesia machines allows you flexibility to address customer tailored combinations as well as an ergonomic and user-friendly workplace for nearly every size of OR.
Scalability among workstation set-up addresses various customer needs and meets spatial conditions of different OR spaces:
— Compact or large trolley, ceiling or wall variants supports good patient access, ergonomic working environment, and low turnaround times with customised workplaces
— Comes with or w/o integrated patient-gas measurement module to offer flexibility and avoid redundant cost for clinics with gas bench monitors
Standardised Drager user interfaces, operating principles, nomenclature, and accessories across other Drager anaesthesia devices and ventilators reduce training efforts, optimise fleet management and reduce tisk of errors
Graphically illustrated walk-through pre-test checklist enables easy and intuitive preparation of the machine for self-test
Fully automated system self-test* (no user interaction needed) enhances operational efficiency and saves staff time for other tasks �
– Auto On** function enables an automatic system test and switching on of the tested device at a defined time that helps to reduce time for start-up
— Ex and import of machine configuration via USB saves manual efforts and time**
— Large work surface, lockable drawer, and additional shelves (optional) for optimal working conditions and supply storage
— Workplace illumination improves readability during Minimally invasive surgery (MIS) cases
— Cable management channels reduce cable clutter, connection failures and cleaning efforts
— Improved manoeuvrability via combination with ceiling supply units simplifies positioning of machine in the OR
– Anaesthetic agent and gas consumption measurements help to analyse potential savings in agent and gas consumption
— Generates a message*** when the maximum period of use of the RFID technology-based accessories (Infinity ID breathing circuit, Infinity ID WaterLock 2 water trap, Infinity ID flow sensors, Infinity ID CLIC absorber) are exceeded to remind personnel about the required replacement of consumables
— Generates message* when the RFID technology-based Infinity ID breathing bag connector or breathing circuit is connected incorrectly and if the Infinity ID CLIC absorber is not firmly connected to avoid potential human errors
— Design flexibility enables different mounting positions of hardware components, e.g. patient monitors, IV pumps, IT hardware and shelves, etc., to offer customised workstation solutions
* variant with integrated Oo monitoring requires weekly calibration of the Og cell. The pre-use checklist has to be performed by the user prior to the self-test.
** this requires software 2.0 or higher
*** with option Infinity ID Accessories Support
Cybersecurity
The Atlan A350/XL anaesthesia machine was designed with security in mind to combat dangerous and damaging cyber-attacks.
We implemented measures considering the NIST security best practices framework.
— Identify: Dedicated documents with security relevant information are provided for asset risk management (e.g. Software Bill of Material, MDS2 Form, comprehensive cybersecurity whitepaper).
— Protect: – A secure boot ensures the integrity of the software running on the device
— Role-based authentication & authorisation prevents unauthorised access to critical settings and data
— Hardened operating system by omitting all unnecessary software components and disabling all unused ports minimises attack surface
— Detect: Security relevant events are detected, logged in a tamper-proof security log file and IT-admin is notified via SNMP traps
— Respond: The system health monitor observes the system load carefully and reacts in case of suspected malicious events, i.e., disable network interface if load is unusually high. �
— Recover: The system can reboot into last good known state if security event is detected. Drager service can restore hard- and software quickly, clinical configuration can be transferred from other devices via USB drive
Atlan was developed as to our secure development lifecycle encompassing:
— Threat analysis to identify vulnerabilities during the development phase
— Automatic code analysis along software development
— Independent 3rd party penetration testing to discover residual vulnerabilities
— Execution only of signed (trusted) code on the device
— Release of patches if relevant vulnerability was detected
– Continuous vulnerability monitoring along the lifecycle of the product